5th How to Diagnose and Treat Acute Leukaemias
27/02/2026
5th How to Diagnose and Treat:
Acute Leukaemias
SAVE THE DATE : February 27 – March 1, 2026 – Mandelieu-La Napoule, France
Chairs: Hervé Dombret, Christoph Röllig, Wendy Stock
#ESHAL2026
Read more
24th annual congress of the SFGM-TC
19/11/2025
24th Annual SFGM-TC Congress on 19, 20 and 21 November 2025 in Geneva, Switzerland.
As every year, several major sessions will punctuate the event:
Patient, donor and caregiver session
GFIC-GM Nursing Day
TEC/data managers day
Educational session
Read more
2nd EDITION LEUKEMIA360 - NOVEMBER 13th 2025 PARIS
13/11/2025

On 13 November 2025, the Pierre Mendès Conference Centre in Paris will host the second edition of LEUKEMIA 360, a unique event bringing together more than 40 research organisations involved in leukaemia and related diseases.
The OPALE Carnot Institute has brought together French haematology experts and international specialists to discuss recent developments in therapeutic and diagnostic solutions for leukaemia. As last year, the event is supported by the National Cancer Institute, joined this year by the ARC Foundation for Cancer Research.
Read more
LSC Editorial Haematologica 17 July 2025
17/07/2025
- Adriana PLESA
- Christophe ROUMIER
Laboratory of Hematology and Flow cytometry, Lyon-Sud Hospital, HCL-CHU Lyon; CRCL INSERM 1052/CNRS 5286, University of Lyon, Hospices Civils de Lyon, Lyon
Haematologica Early view Jul 17, 2025 https://doi.org/10.3324/haematol.2025.288003
CRAs Day at SFH 2025
03/04/2025
Newsletter Force Hemato
13/01/2025
66th ASH Annual Meeting
07/12/2024
The 66th ASH Annual Meeting and Exposition will take place December 7-10, 2024, in San Diego, California,
66th ASH Annual Meeting & Exposition - Hematology.org
Read more
ASH2024 MRDflow BIG-1
07/12/2024
Flow MRD Monitoring Combining LAIP/Dfn and CD34+ CD38 LSCs Is a Strong Predictor of Outcome in Adult AML Independently of the ELN 2022 risk
First Results from the Multicentric Acute Leukemia French Intergroup MRD Flow Network (BIG 1 Study)
SGFM TC CONGRESS 2024
20/11/2024
LEUKEMIA 360
13/11/2024
On 13 November, the Pierre Mendès Conference Centre in Paris will host LEUKEMIA 360, a unique event dedicated to public-private collaborations in the research and development of therapeutic and diagnostic solutions for leukaemia and related diseases.
The OPALE Carnot Institute brought together French haematology experts and international specialists to discuss recent developments in therapeutic and diagnostic solutions for leukaemia. Stéphanie Fugain, President of France's leading patient organisation, will also bring the perspective of leukaemia patients.
All the information and the programme are available on the Home - Leukemia 360 event Home - Leukemia 360
EHA congress 2024, Madrid from 13 to 16 June 2024
13/06/2024
SFGM-TC 2022 CONGRESS
15/09/2023

- Congress site : https://www.sfgmtc-congres.fr/
- program : https://www.sfgmtc-congres.fr/congres-de-la-sfgm-tc
- Inscription : https://www.sfgmtc-congres.fr/inscriptions
Poster ASH2022 MRDflowAML ALFA
10/12/2022
AML MRD by multiparameter flow cytometry using LAIP/Dfn and LSC: Methodological aspects in a multicentric study of the French-Flow MRD AML ALFA Network
Read more« 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party »
02/03/2022
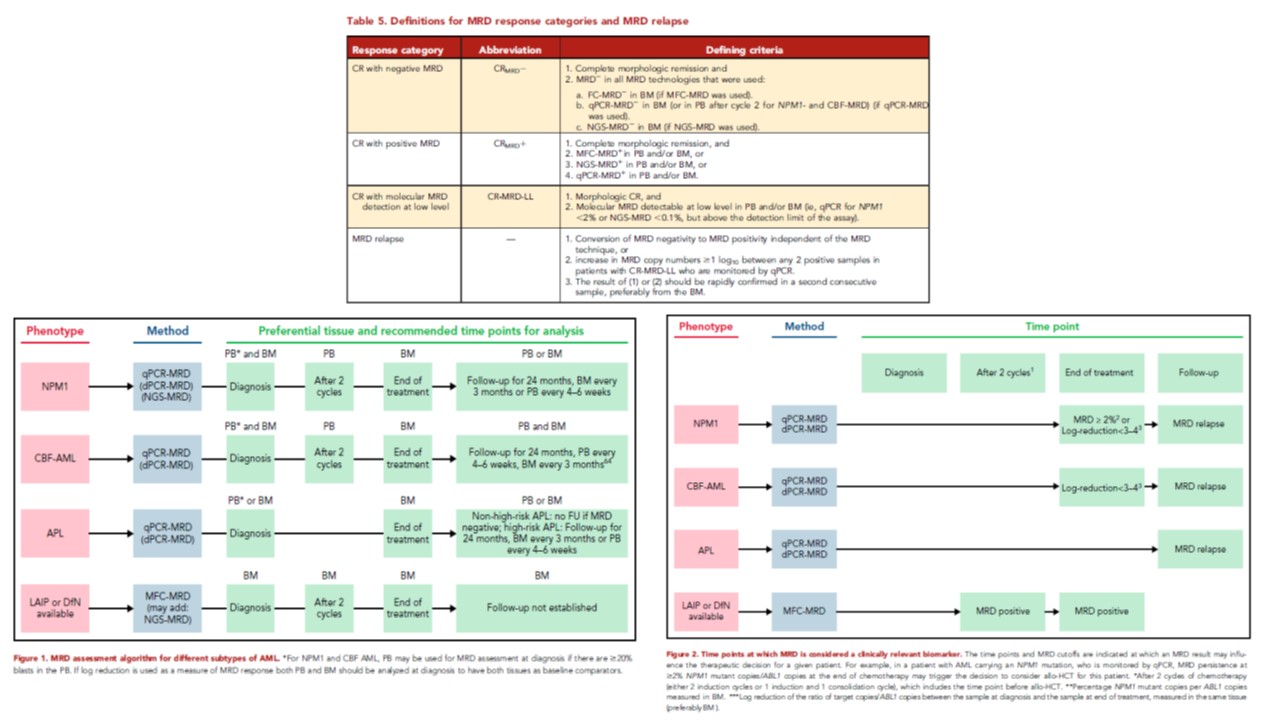
Leukemia Abberant ImmunoPhenotype / Different of normal» (MRD LAIP/Dfn) et « Leukemia Stem Cells » (MRD-LSC).
02/03/2022
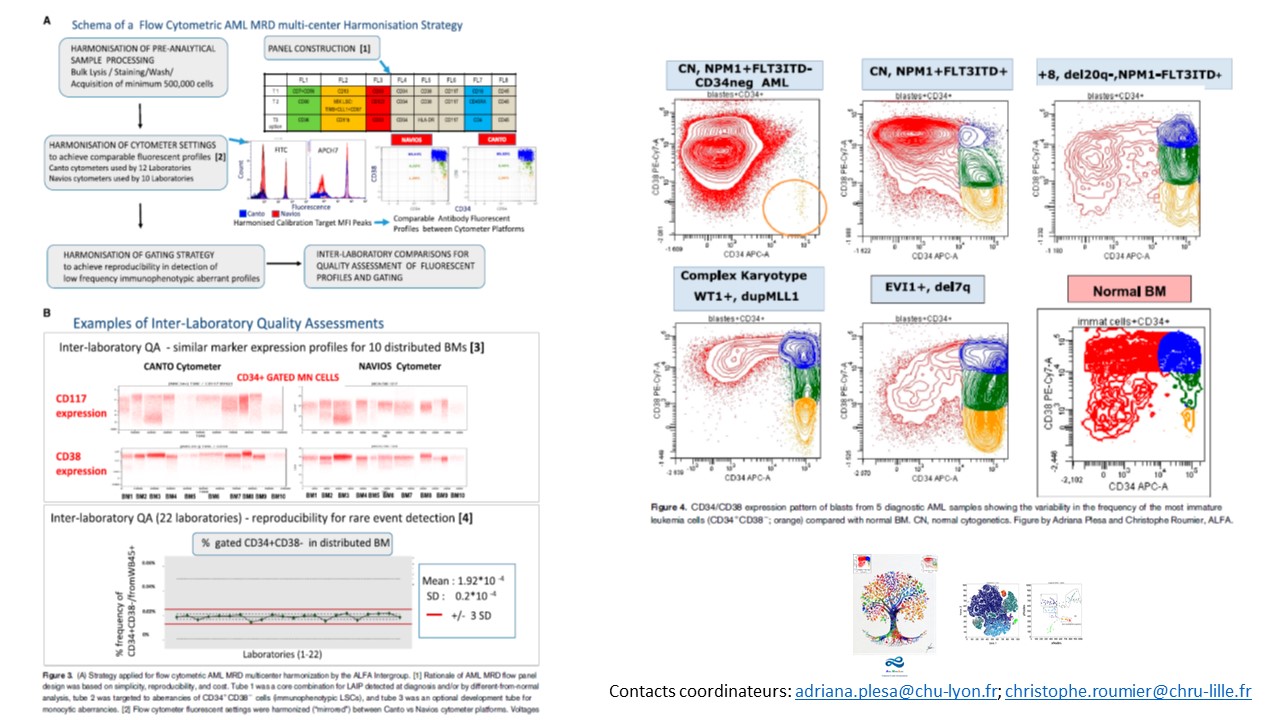
AML MRD by multiparameter flow cytometry using LAIP and LSC: Methodological aspects in a multicentric study of the French AML Intergroup
02/03/2022
News – ALFA contribues to the optimization of allogeneic hematopoietic stem cell transplantation (HSCT) indications in first remission in AML patients.
17/03/2021
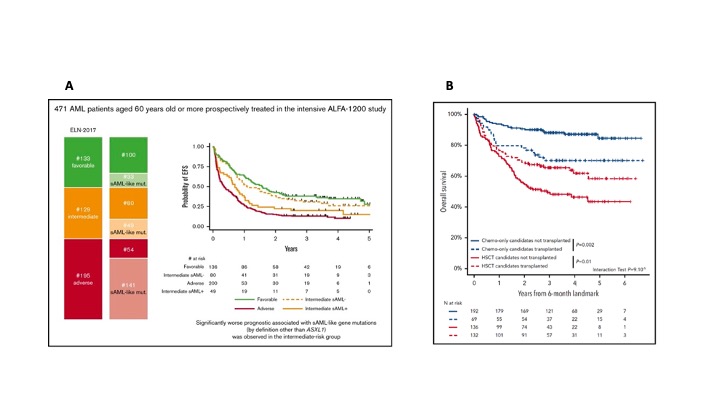
In two recent studies conducted in older and younger AML patients, respectively, and through the integration of more patient/disease-related annotations in the decision-making (including the mutational profiles), the ALFA group proposes significant refinements in the indications of allogeneic HSCT in first remission.
Currently, HSCT indications are based on the European LeukemiaNet (ELN) risk classification, which includes conventional cytogenetics and mutations in the six following genes: NPM1, CEBPA, FLT3, ASXL1, RUNX1 et TP53. In the favorable AML subtypes, like core binding factor (CBF) AML or AML with NPM1 gene mutation, measurable residual disease (MRD) levels after induction and/or consolidation might also be taken into account for this important clinical decision: transplant or not transplant?
Older AML
In a first paper published in May 2020 in Blood Advances, C. Gardin and coll. studied the incidence and prognostic impact of so-called “secondary AML-like (sAML-like)” mutations, as described by R. Coleman Lindsley and coll. These mutations include not only the ASXL1 gene, but also SRSF2, SF3B1, U2AF1, ZRSR2, EZH2, BCOR et STAG2. Interestingly, this molecular definition allowed classifying as "having a secondary AML” almost half of older AML patients aged 60 years old or more included in the prospective ALFA-1200 study (48% of the entire cohort, 38% of intermediate ELN-risk AML patients), even if only 15% had prior clinically identified hematological disorders. In the intermediate ELN-risk group, the presence of sAML-like gene mutations allowed discriminating a subset of patients almost similar to adverse-risk AML patients in terms of outcome, while those without these mutations had an event-free survival close to that observed in favorable-risk AML patients (Figure A). In terms of overall survival, allogeneic transplantation in first remission benefited to the former subgroup (adverse + intermediate sAML+), while not to the latter subgroup (favorable + intermediate sAML-).
Younger AML
In a more recent publication (Blood Jan 2021) based on the younger AML ALFA-0702 study, L. Fenwarth, R. Itzykson and coll. enriched the ELN-2017 classification through the integration of items from a Knowledge Bank (KB), as published by M. Gerstund and coll. in 2017, as well as NPM1-MRD levels in the subset of patients with NPM1-mutated AML. The important result of this study was that this integration allowed substantially improving the prognostic value of the ELN-2017 classification. The combination of these two scores (ELN-2017 + KB) was associated with an impressive prediction of the effect of allogeneic HSCT in first remission. Even if this new approach requires validation in other future prospective studies, it is amazing to see how transplantation might benefit to 50% of the patients, while being detrimental in the other 50% (Figure B).
State of play of the AML MRD LSCflow protocol within the ALFA clinical trial: study of new LSC markers and integration of the unsupervised FlowSom and TSNE approach in data analysis
04/11/2020
The new institute Carnot OPALE unites French partnership research on leukemia
10/01/2020

The newly created institute Carnot OPALE gathers the most important research and development French teams working in the field of leukemia and related disorders. This makes OPALE the reference partner for all companies developing innovate diagnostic, therapeutic and follow-up tools for patients with these diseases.
Leukemias and related disorders are the more devastating blood cancers. Their incidence is increasing, with 2.3 million people currently suffering from these diseases. They also represent the most frequent childhood cancers.
The institute Carnot OPALE (Organization for Partnership Research in Leukemia) structures all the preclinical and clinical French research forces, with the aim to present a comprehensive R&D offer to industrial companies interested in this medical field. OPALE belongs to the 39 institutes Carnot labeled in February 2020 by the French Ministry for Higher Education, Research and Innovation for a 4-year period of time.
The institute, member of the Institute Carnot Association and the FINDMED consortium, a research partner for medicinal product SME, gathers 17 research teams, 7 cooperative groups and 4 R&D platforms,1 covering the entire R&D chain as well as the medical and industrial valorization processes.
Basic, translational and clinical research fields of OPALE include:
• New therapeutic target discovery
• Innovative drug development, including cell and gene therapies
• Hospital diagnosis and care
• Clinical trials
• Real-world and long-term follow-up
• Startup incubation
With approximately 700 full-time equivalent employees and an annual 7.2 M€ budget of partnership research, the ultimate goal of OPALE is to define new curative, ambulatory, less toxic and financially sustainable treatment options for all patients with leukemia and related disorders during the next 10-year period.
Website: https://www.opale.org
OPALE entities:
- Cooperative groups for clinical research:
• The Organization for Partnerships in Leukemia (OPALE)
• Acute Leukemia French Association (ALFA),
• Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL),
• French Innovative Leukemia Organization, (FILO),
• Groupe Francophone des Myélodysplasies (GFM),
• French Intergroup Myeloproliferative Disorders (FIM),
• Société Française de Lutte contre les Cancers et Leucémies de l’Enfant et de l'Adolescent (SFCE),
- Research teams:
• Centre National de Médecine de Précision sur les Leucémies THEMA (UMR-944, UMR-1131, UMR-1160, UMR-976, EA-3518, Institut de Recherche Saint-Louis, Université de Paris),
• Centre de Recherche en Cancérologie de Marseille (UMR-1068 ; Institut Paoli-Calmettes, Université Aix-Marseille),
• Centre de Recherche en Cancérologie de Toulouse (UMR-1037 ; Oncopôle, Université de Toulouse III),
• Université de Bourgogne Franche-Comté (UMR-1098 ; Besançon),
• Institut de Radiobiologie Cellulaire et Moléculaire (UMR-1274, CEA Fontenay-aux-Roses),
• Institut IDMIT (UMR-1184 ; CEA Fontenay-aux-Roses),
• Institut Cochin (UMR-1016 ; Université de Paris),
• Gustave Roussy (UMR-1170 ; Université Paris Saclay)
• Centre de Recherche Saint-Antoine (UMR-938 ; Sorbonne Université),
• Centre de Recherche Jean-Pierre Aubert (UMR-1172 ; Université de Lille),
• Centre Méditérranéen de Médecine Moléculaire (INSERM-1065 ; Université Nice Cote d’Azur),
• Université de Bordeaux (UMR-1035),
• Université de Tours (ERL7501 LNOX),
- R&D platforms:
• Plateforme Meary de Thérapie Cellulaire et Génique / CIC-BT (Paris Saint-Louis),
• Plateforme de Thérapie Cellulaire et Génique / CIC-BT (Marseille IPC),
• Plateforme Meary de R&D Génomique et Bioinformatique (Paris Saint-Louis),
Contacts:
Hervé Dombret, M.D./ Professor of Hematology, Institut de Recherche Saint-Louis, Hôpital Saint-Louis (AP-HP), OPALE Chairman
Pascal Deschaseaux, M.D./ OPALE General Director / pascal.deschaseaux@opale.org
